Mi-Trial For Industry
The Clinical Trial Companion App
Mi-Trial supports better clinical trial delivery by keeping participants informed, engaged, and on-track. Through intelligent scheduling and timely communication, Mi-Trial helps trial teams reduce operational burden, minimise missed visits, and improve protocol adherence.
Mi-Trial was conceived by clinical researchers working on and delivering clinical trials. Professors Alex Horsley and Jacky Smith are both clinical academics working in Respiratory medicine (cystic fibrosis and cough research respectively) and between them have led and
designed dozens of clinical trials. The other partner is the SME ELAROS.
ELAROS is a Sheffield-based developer of medical apps, already partnered with a number of different NHS organisations and currently rolling out autonom-e, a customisable digital solution for the remote assessment, triage, monitoring, management, rehabilitation, and education of patients with a range of long-term conditions built on the C19-YRS Long COVID symptom app.
Developed in partnership with the Medicines Evaluation Unit (MEU).
The Mi-Trial team of clinical researchers, clinical academics and a digital health company provide a unique combination of experience to this project. The app has been developed over the last 3 years by the partners in the Mi-Trial Company (a University of Manchester spin-out).
Why Industry Partners Choose Mi-Trial
-
Custom-branded trial app
Create a dedicated app for your study - branded to your trial, sponsor, or CRO. This enhances engagement, improves site adoption, and strengthens your digital identity.
-
Scalable architecture
Mi‑Trial supports multiple centres, large participant numbers, and long‑running studies. Additional instances can be deployed for separate trials or sponsors.
-
Compliance and security built in
Mi‑Trial supports external penetration testing, secure AWS hosting, and governance processes aligned with NHS and university standards.
-
Faster and more affordable
Mi‑Trial offers a validated, ready‑to‑deploy alternative at a fraction of the cost.
Tailored Solutions
- Standardise digital tools across multiple studies.
- Offer participants a modern, app‑based participant experience.
- Reduce development timelines.
- Improve data quality and protocol adherence.
- Provide white‑labelled versions of the platform for CRO‑specific branding.
Product Screenshots (Web Portal)
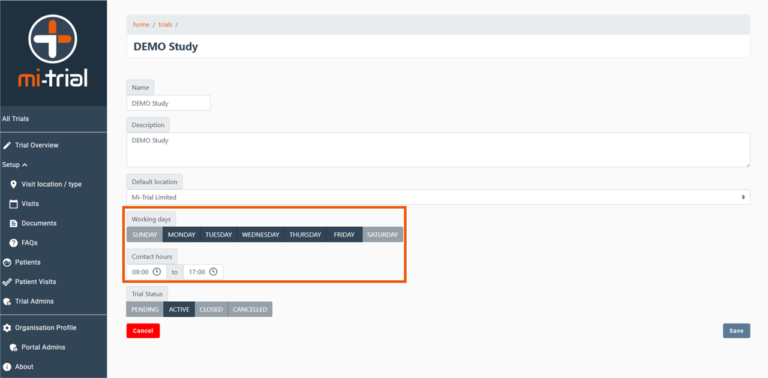
Trial Setup view, where the trial organisation configures settings. Working days and contact hours feed into the scheduling algorithm to help determine the most time-efficient visit times, whilst maintaining protocol and valid date ranges.
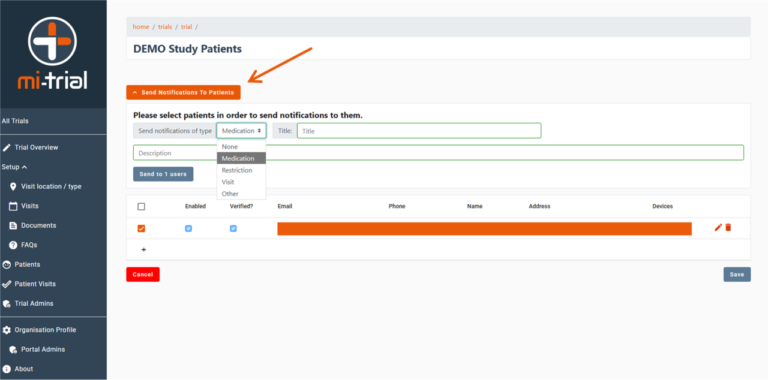
Patients menu in Portal mode, where the trial organisers can send bespoke / custom notifications to specific patients.
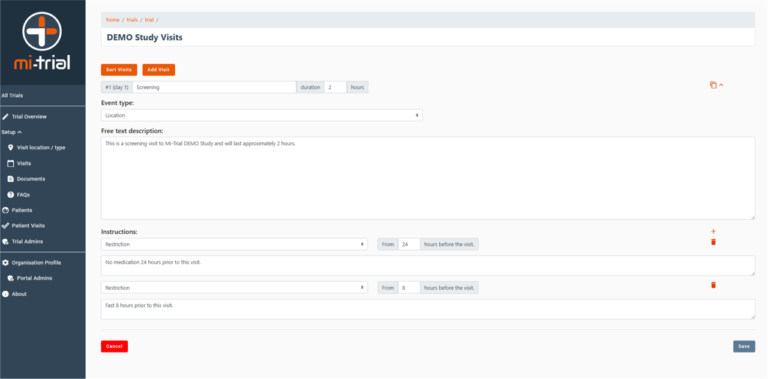
Interface to setup and configure study Visits. The system is designed to be flexible and enable various study structures to be set up. If a patient visit occurs in a date which breaches the boundaries defined here, the portal administrator will receive a notification of this to either amend the dates or check / approve the out of bounds date.
Product Screenshots (Web App)
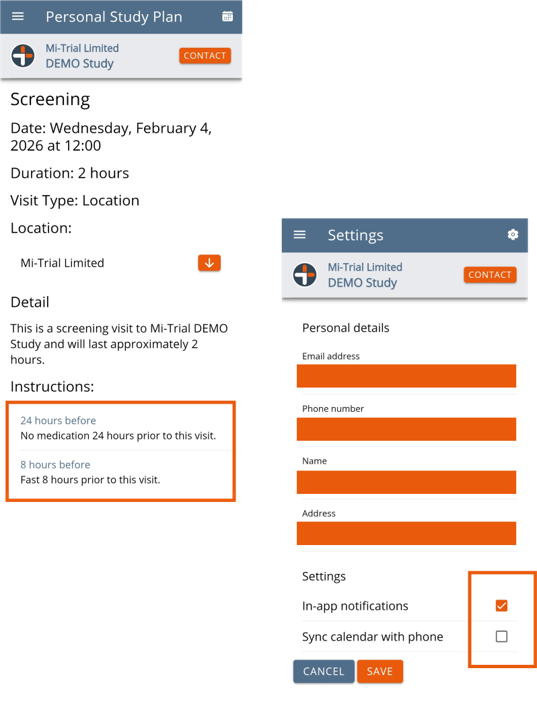
Participant view of an event in their Personal Study Plan. Note the two instructions which will also populate the Notifications page and also send as push-notifications if the participant has these enabled. SMS / Email notifications functionality can be added.
A web app version is also available to access off the mobile.